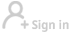Leading vaccine maker seeks market approval

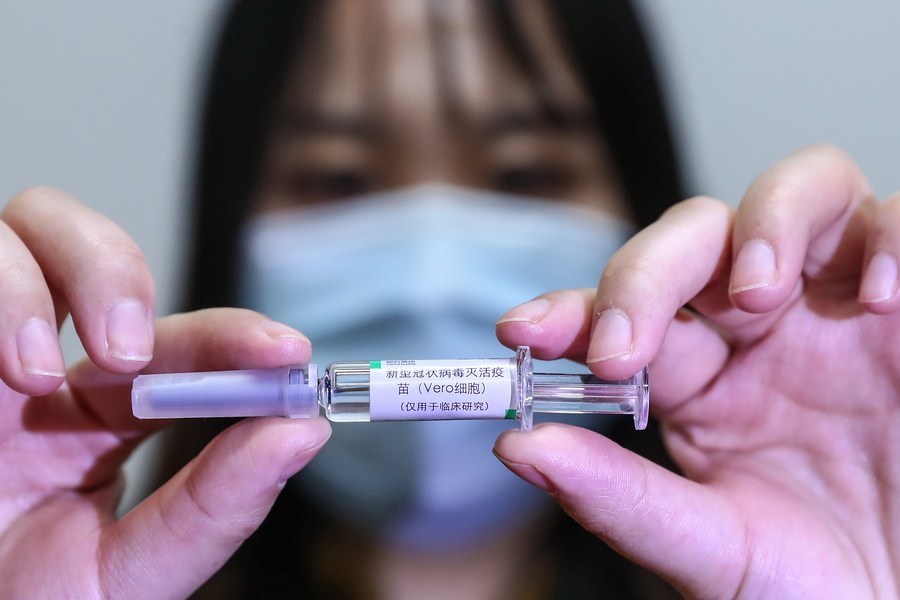
Leading COVID-19 vaccine developer Sinopharm has submitted a market approval application to China's top market regulator, Xinhua News Agency quoted the company's Deputy General Manager Shi Shengyi as saying on Wednesday.
The Chinese company has two experimental COVID-19 vaccines - both of which are the inactivated type - undergoing Phase 3 clinical trials overseas.
The Xinhua report did not reveal details about the application.
Liu Jingzhen, chairman of Sinopharm, said previously that hundreds of thousands of people in China have been injected with either one of the vaccines through emergency authorization, and 56,000 of them have traveled abroad after inoculation.
No severe adverse effects have been observed among them, and none of those now living overseas has been infected with the virus, he said.
Regarding the overseas trials, Liu said nearly 60,000 volunteers from 10 countries have been enrolled in the program, and preliminary results are satisfactory.
The company's production capability is expected to exceed 1 billion doses by the end of next year.
China now has five COVID-19 vaccines in Phase 3 clinical trials in foreign countries, making it a front-runner in the global race to create a safe, effective vaccine.
- Over 1,600 delegates attend regenerative medicine conference in Wuhan
- Beijing steps up winter heating preparations
- 2025 Xinjiang Tacheng Baktu Forum opens, strengthening regional cooperation
- First satellite produced in Xiong'an marks industry milestone
- New endoscopic tech for complex spine surgery attracts global experts
- ASEAN+3 STEM competition launches in Chongqing